Answer: The compound that has element ratio of 1 : 1 : 1 is KOH
Step-by-step explanation:
Mole ratio is defined as the ratio of number of moles of the two elements combining to form a compound.
In a chemical compound, the subscripts represents the number of moles of elements in a compound.
For the given chemical reaction:
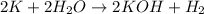
 has two elements, hydrogen and oxygen and is present in the ratio of 2 : 1
has two elements, hydrogen and oxygen and is present in the ratio of 2 : 1
KOH has three elements, potassium, hydrogen and oxygen and is present in the ratio of 1 : 1 : 1
Hence, the compound that has element ratio of 1 : 1 : 1 is KOH