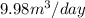Answer : The volume of air at STP that an individual breathes in one day is
Explanation :
Combined gas law is the combination of Boyle's law, Charles's law and Gay-Lussac's law.
The combined gas equation is,
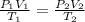
where,
 = initial pressure of air =
= initial pressure of air =
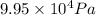
 = final pressure of air at STP = 1 atm =
= final pressure of air at STP = 1 atm =

 = initial volume of air =
= initial volume of air =
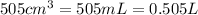
 = final volume of air at STP = ?
= final volume of air at STP = ?
 = initial temperature of air =
= initial temperature of air =
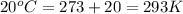
 = final temperature of gas =
= final temperature of gas =
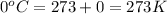
Now put all the given values in the above equation, we get:
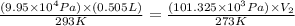
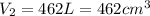
The volume for a single breath at STP is,
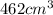
Now we have to determine the volume of air at STP that an individual breathes in one day.
To determine the volume of air inhaled in one day, use the number of breaths per minute. Now convert minutes into hours. Then, convert hours into days. Finally, convert cubic centimeters into cubic meters, we get:
conversion used :
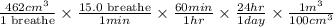
=
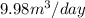
Therefore, the volume of air at STP that an individual breathes in one day is