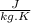Answer:
4000
Step-by-step explanation:
The temperature of 0.1 kg of liquid rises from 25°C to 50°C in 300 sec. Energy of 13,600 J was supplied during this time. Appartus was losing energy at the rate of 12 J/sec.
Let us assume the Specific heat capacity as
 .
.
As there is no state change from liquid to gas, only Specific heat capacity is involved. Also, work done is approximately zero because volume does not change much. So,
Energy gained = Energy required to rise the temperature
Energy gained by liquid =
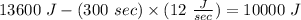
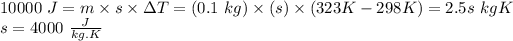
∴ Specific heat capacity of liquid = 4000