Answer:Xenon
Step-by-step explanation:
The average atomic mass of an element is defined as the sum of the masses of its isotopes,each multiplied by their natural abundances.
Option A:
Argon has a average atomic mass of
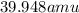
Option B:
Xenon has a average atomic mass of
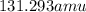
Option C:
Krypton has a average atomic mass of
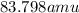
Option D:
Neon has a average atomic mass of
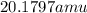
So,Xenon has the highest average atomic mass.