Answer:
0.15625M
Step-by-step explanation:
When you mix two liquids of the same solute and solvent but with different concentration and amounts then the resulting solutions molarity is given by
C
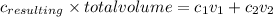 .
.
in the above case given,
c1=0.1M
v1=25mL
c2=0.175M
v2=75mL
total volume=v1+v2=25+75=100mL.
so substituting thse values in the above equation gives C_{equivalent}[/tex]=15.625\100=0.15625M