Answer:
Final boiling point of water is 376·06 K
Step-by-step explanation:
As NaCl is a non-volatile solute so by the addition of non-volatile solute to a pure solvent will increase its boiling point
Formula for the change in boiling point of the solution is
ΔT
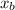 = i × K
= i × K
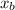 × m
× m
where
ΔT
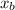 is the change in boiling point of the solution
is the change in boiling point of the solution
i is the vant Hoff factor which is the number of particles per single molecule of the solute
K
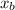 is the cryoscopic constant which only depends on the properties of the solvent and not on the solute
is the cryoscopic constant which only depends on the properties of the solvent and not on the solute
m is the molality of the solute
in this case molality of the NaCl is 3÷1 =3 mole/kg
By the formula
ΔT
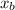 = 2×0·51×3=3·06 K
= 2×0·51×3=3·06 K
Boiling point of the pure solvent( in this case it is water) is 373 K
Final boiling point of the water = ΔT
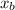 + 373
+ 373
= 3·06+373
∴ Final boiling point of the water = 376·06 K