Answer: 82 moles
Step-by-step explanation:
Combustion is a type of chemical reaction in which fuel is reacted with oxygen to form carbon dioxide and water.
The balanced equation for combustion of glucose is:
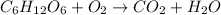
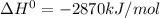
Thus 2870 kJ of energy is released by combustion of 1 mole of glucose
Given : 1 mole of hypothetical compound X use 35.1 kJ of energy
If 35.1 kJ of energy produces = 1 mole of hypothetical compound X
2870 kJ of energy produce =
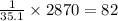 moles of hypothetical compound X
moles of hypothetical compound X
Thus 82 moles of hypothetical compound X could theoretically be generated.