Answer : The pressure of the sample of gas is 959.1 torr
Explanation : Given,
Atmospheric pressure = 1.23 atm = 934.8 torr
conversion used : (1 atm = 760 torr)
Gauge pressure or pressure difference = 24.3 torr
As we know that:
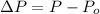
where,
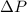 = Gauge pressure or pressure difference
= Gauge pressure or pressure difference
P = pressure of the sample of gas
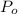 = Atmospheric pressure
= Atmospheric pressure
Now put all the given values in the above expression, we get:
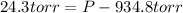
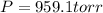
Therefore, the pressure of the sample of gas is 959.1 torr