Answer:
V = 4.48 l
Step-by-step explanation:
Standard temperature and pressure is at the temperature at 273 K (0 C) and pressure of 1 atm (101325 N/m2 or 760 mmHg).
The general gas law,
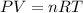
Considering the first condition
n = 1.00 mole,
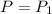 ,
,
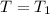 , R = Constant, V = 22.4 l
, R = Constant, V = 22.4 l
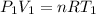 ---------------------Eqn 1
---------------------Eqn 1
n = 0.200 mole,
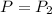 ,
,
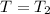 , R = Constant, V = ??
, R = Constant, V = ??
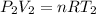 ---------------------Eqn 2
---------------------Eqn 2
Under the same condition,
 =
=
 ,
,
 =
=

Dividing Eqn 1 by Eqn 2
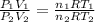
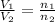
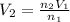
V =
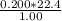
V = 4.48 l