Answer:
V₂= 1 L
Step-by-step explanation:
Given that
Volume occupies V₁= 6 L
Initial pressure = P₁
Initial temperature = T₁
The final pressure =P₂ = 2 P₁
Final volume =V₂
Final temperature = T₁/3
As we know that equation for ideal gas
P V = m R T
P=pressure, V=volume, T=temperature
m=mass ,R=gas constant
Now from mass conservation
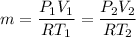
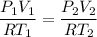
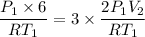
6 = 3 x 2 V₂
V₂= 1 L
So the final volume will be 1 L