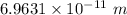Answer:
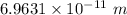
Step-by-step explanation:
I = Moment of inertia =
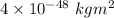
m = Mass of two atoms = 2m =
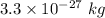
r = distance between axis and rotation mass
Moment of inertia of the system is given by
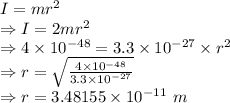
The distance between the atoms will be two times the distance between axis and rotation mass.
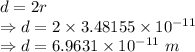
Therefore, the distance between the two atoms is