Answer:
Total concentration of ions present in the final solution is 0.0726 M.
Step-by-step explanation:
Mass of silver nitrate = 54.0 g
Moles of silver nitrate =
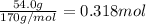
Volume of the solution made = V = 350.0 mL = 0.350 L
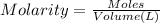
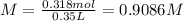
After dilution of 10.00 mL 0.9227 M solution.
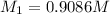
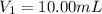
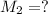
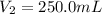

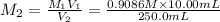
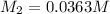
Concentration of silver nitrate after dilution = 0.0363 M
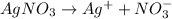
![[AgNO_3]=[Ag^+]=[NO_3^(-)]=0.0363 M](https://img.qammunity.org/2020/formulas/chemistry/college/h01e63ii3hl6q7zwilfvo8dcwzqizzg9f7.png)
Total concentration of ions present in the final solution:
![[Ag^+]+[NO_3^(-)]=0.0363 M+0.0363 M=0.0726 M](https://img.qammunity.org/2020/formulas/chemistry/college/n7mpywfjibbv8zhzjsya5ezrk0a5hwtaf0.png)