Answer:
1) Enthalpy of reaction per gram of quick lime or calcium oxide is -6.25 kJ/g.
2)
 kilo Joules of energy is produced when 1 Tonn of slaked lime is produced.
kilo Joules of energy is produced when 1 Tonn of slaked lime is produced.
Step-by-step explanation:
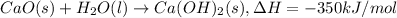
Enthalpy of reaction = ΔH = -350 kJ/mol
1 mole of calcium oxide = 56 g/mol
1 ) Enthalpy of reaction per gram of quick lime or calcium oxide:
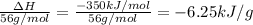
2) Mass of slake lime = 1 Tonn = 1,000,000 g
Moles of slaked lime =
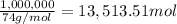
According to reaction, on formation of 1 mol of slaked lime 350 kilo Joules of energy is produced.
Then energy produced when 13,513.51 moles of slaked lime is produced:
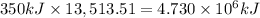
 kilo Joules of energy is produced when 1 Tonn of slaked lime is produced.
kilo Joules of energy is produced when 1 Tonn of slaked lime is produced.