Answer:
0.000324 kg/m²h
Step-by-step explanation:
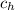 = High concentration = 2 kg/m³
= High concentration = 2 kg/m³
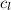 = Low concentration = 0.2 kg/m³
= Low concentration = 0.2 kg/m³
 = One Length = 0
= One Length = 0
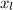 = Other Length = 2 mm
= Other Length = 2 mm
D = Diffusion coefficient =
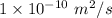
The flow rate through a wall from Fick's Law is given by
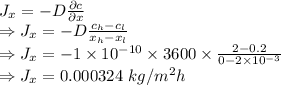
The flow rate through the wall is 0.000324 kg/m²h