Answer:
The specific heat capacity of the unknown metal is 1.04 J/g°C.
Step-by-step explanation:
Let the specific heat capacity of the unknown metal be
 .
.
Given:
Mass of the metal is,
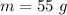
Initial temperature of the metal is,
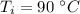
Volume of water is,
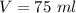
Specific heat capacity of water is,
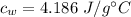
Initial temperature of water is,
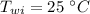
Final temperature of the system is,
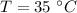
We know that density of water is equal to 1 g/ml.
Mass is given as the product of density and volume.
Therefore, mass of water is given as:
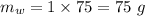
Now, fall of temperature of the unknown metal is given as:
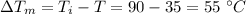
Rise of temperature of water is given as:

Now, as per conservation of energy,
Heat lost by metal = Heat gained by water
⇒
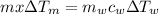
Plug in all the given values and solve for
 . This gives,
. This gives,
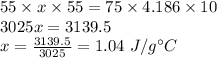
Therefore, the specific heat capacity of the unknown metal is 1.04 J/g°C.