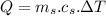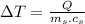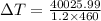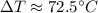Answer:
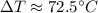
Step-by-step explanation:
Given:
Volume of water,
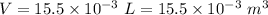
initial temperature of water,
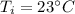
final temperature of water before forming steam,
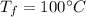
mass of hot skillet,
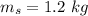
Usually skillets are made of cast iron and cast iron has a specific heat capacity of :

Specific heat of water,
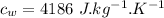
Latent heat of vaporization of water,

Now, mass of water:
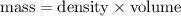
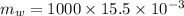

Quantity of heat absorbed by the water of 23 degree Celsius to a point just before steaming:
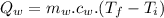
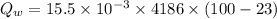
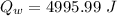
Quantity of heat absorbed by the water to vapourize:
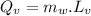
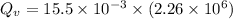
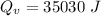
∴Total heat lost by the skillet:
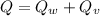
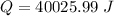
For change in temperature: