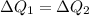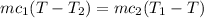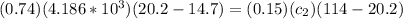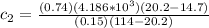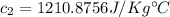To perform this exercise it is necessary to use the theory of the first Law of Thermodynamics, in relation to Heat Capacity. If one adds heat to an object, its temperature usually increases (exceptions include at a state boundary, for example when a liquid boils). In many cases the temperature change is linear in the amount of heat added. We define the heat capacity C of an object from the relation:
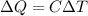
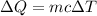
Where
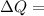 Heat that flows into system
Heat that flows into system
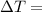 Temperature
Temperature
c = Specific heat
m = Mass
From our values we have the exchange of heat of the material and of the water, that is,
For the water:
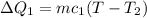
Where,
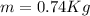
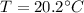
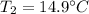
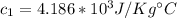
For the Material:
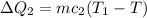
Where,
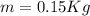
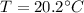
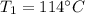
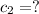
For conservation of energy in balance we have to,