Answer:
There are 12 sigma bonds and 3 pi bonds in benzene
Step-by-step explanation:
- Molecular formula of benzene is
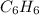 .
.
- Here six carbon atoms are joined together through alternative single and double bond forming a six membered cyclic ring.
- Six hydrogen atoms are attached to six carbon atoms through a single bond.
- A single bond represents a sigma bond. A double bond consists of a sigma bond and a pi bond.
- In the Lewis structure of benzene, there are three C-C single bonds, three C=C double bonds and six C-H single bond.
- Hence there are 12 sigma bonds and 3 pi bonds in benzene
- Lewis structure of benzene is given below