Answer:
Before we start answering, the appropiate equation for the energy of an ionic bond should be written:
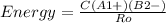
Where A1+, B2- are the charges of the ions and Ro is the interionic distance (the sum of the radii of the anion and cation). C is a constant depending on the ionic structure.
Step-by-step explanation:
Examining the equation above, we have the following scenarios:
a) Doubling the radius of A1 would mean a decrease on the energy of the ionic bond.
b) Tripling the charge of A1 would also triple the energy of the ionic bond
c)Doubling the charges on A1 and B2 would quadruple the energy of the ionic bond.
d)Decreasing the radii of A1 and B2 to half their original values would double the energy of the energy bond.