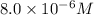Answer:
Ferric ions left in the solution at equilibrium is
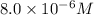 .
.
Step-by-step explanation:
Moles of ferric nitrate in 0.700 L = n
Volume of the solution = V = 0.700 L
Molarity of ferric nitrate = M = 0.00150 M
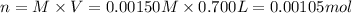
Moles of potassium thiocyanate in 0.700 L = n'
Volume of the potassium thiocyanate solution = V' = 0.700 L
Molarity of potassium thiocyanate = M' = 0.200 M
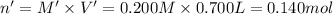
Molarity of ferric ions after mixing :
1 mol of ferric nitrate gives 1 mol of ferric ions.Then 0.00105 mol ferric nitrate will :
Moles of ferric ions = 0.00105 mol
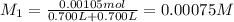
Molarity of thiocyanate ions after mixing :
1 mol of potassium thiocyanate gives 1 mol of thiocyanate ions.Then 0.140 mol potassium thiocyanate will give:
Moles of thiocyanate ions = 0.140 mol
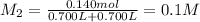
Complex equation:
![Fe^(3+)+SCN^-\rightleftharpoons [Fe(SCN)]^(2+)](https://img.qammunity.org/2020/formulas/chemistry/college/53qw91hbkaf08itdxk5g1f6b465zchmphy.png)
0.00075 M 0.1 M 0
At equilibrium:
(0.00075 M -x) (0.1 M-x) x
The formation constant of the given complex =
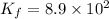
![K_f=([[Fe(SCN)]^(2+)])/([[Fe^(3+)]][SCN^(-)])](https://img.qammunity.org/2020/formulas/chemistry/college/nc5m4zsh4zz00fmwz1es83fnmgjp0sba83.png)
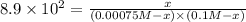
Solving for x:
x = 0.000742 M
Ferric ions left in the solution at equilibrium :
= (0.00075 M -x) = (0.00075 M - 0.000742 M)=