Answer:
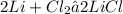
Step-by-step explanation:
In the given equation 1 molecule of Lithium reacts with 1 molecule of chlorine gives Lithium Chloride as a product.
For balancing an equation the number of atoms of each element of reactant should be equal to the number of atoms of each element of product.
Here one atom of Lithium reacts with 2 atom of of Chlorine and produces Lithium Chloride.
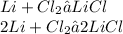
For balancing we have to multiply Lithium by 2 and Lithium Chloride by 2.
So after balancing the number of atoms of 2 molecule of Lithium is 2 and of Chlorine is 2 and of the product Lithium Chloride is also 2.
Thus the given equation is balanced.