Answer:
I = 1010 kJ/mol
Step-by-step explanation:
In PES experiment mercury vapor is irradiated with ultraviolet light of wavelength 58.4 nm.
Energy of the incident photon
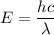
h is planks constant = 6.626 × 10⁻³⁴ J.s
c is speed of light = 3 x 10⁸ m/s
λ = wavelength
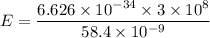
E = 3.40 x 10⁻¹⁸ J
Energy in eV
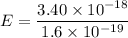
E = 21.2 eV
first ionization energy of mercury is the difference in the kinetic energy of the ejected electron to the energy of the incident photon.
I = 21.2 eV - 10.75 eV
I = 10.45 eV
I = 10.45 x 1.6 x 10⁻¹⁹
I = 1.67 x 10⁻¹⁸ J
now,
I = (1.67 x 10⁻¹⁸ x 6.022 x 10²³) J/mol
I = 10.10 x 10⁵ J/mol
I = 1010 kJ/mol