Answer:
Step-by-step explanation:
The atomic number of Se is 34
Its electronic configuration is the distribution of electrons of an atom in the atomic orbitals
The electronic configuration of Selenium (Se) is
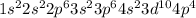
Note that 4s has less energy than 3d so it is written before