Answer:
The speed of the steam is 477.4 m/s.
Step-by-step explanation:
Given that,
Temperature
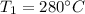
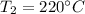
Pressure
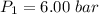
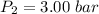
Specific enthalpy of steam at 280°C = 3020 kJ/kg
Specific enthalpy of steam at 220°C = 2906 kJ/kg
We need to calculate the speed of the steam
Using balance equation
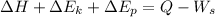

Here,
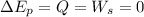
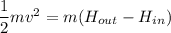
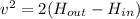
Put the value into the formula
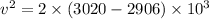
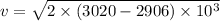
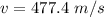
Hence,The speed of the steam is 477.4 m/s.