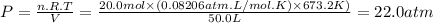Answer:
c) 22
Step-by-step explanation:
Let's consider the following balanced equation.
N₂(g) + 3 H₂(g) ----> 2 NH₃(l)
According to the balanced equation, 34.0 g of NH₃ are produced by 1 mol of N₂. For 170 g of NH₃:
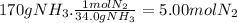
According to the balanced equation, 34.0 g of NH₃ are produced by 3 moles of H₂. For 170 g of NH₃:
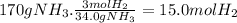
The total gaseous moles before the reaction were 5.00 mol + 15.0 mol = 20.0 mol.
We can calculate the pressure (P) using the ideal gas equation.
P.V = n.R.T
where
V is the volume (50.0 L)
n is the number of moles (20.0 mol)
R is the ideal gas constant (0.08206atm.L/mol.K)
T is the absolute temperature (400.0 + 273.15 = 673.2K)