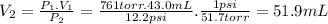Answer:
The volume in Denver is 51.9 mL.
Step-by-step explanation:
The gas initially occupies a volume V₁ = 43.0 mL at a pressure P₁ = 761 torr. To find the volume V₂ at a pressure P₂ = 12.2 psi, when temperature is unchanged, we can use Boyle's law.
P₁.V₁ = P₂.V₂
We need the relation 1 psi = 51.7 torr.