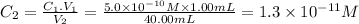Answer:
1.3 × 10⁻¹¹ M
Step-by-step explanation:
We are going to do 4 successive dilutions. In each dilution, we will apply the dilution rule.
C₁.V₁=C₂.V₂
where,
C₁ and V₁ are concentration and volume of the initial state
C₂ and V₂ are concentration and volume of the final state
First dilution
C₁ = 3.1 × 10⁻⁵ M V₁ = 1.00 mL C₂ = ? V₂ = 40.00mL
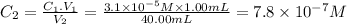
Second dilution
C₁ = 7.8 × 10⁻⁷ M V₁ = 1.00 mL C₂ = ? V₂ = 40.00mL
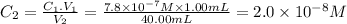
Third dilution
C₁ = 2.0 × 10⁻⁸ M V₁ = 1.00 mL C₂ = ? V₂ = 40.00mL
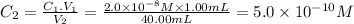
Fourth dilution
C₁ = 5.0 × 10⁻¹⁰ M V₁ = 1.00 mL C₂ = ? V₂ = 40.00mL