Answer:
Half-life for the reaction is 1.92s
Step-by-step explanation:
Integrated rate equation for the given second order reaction is-
![(1)/([A]_(t))=kt+(1)/([A]_(0))](https://img.qammunity.org/2020/formulas/chemistry/college/f6tk8nmdymwyw6finlgc4dsomzqthn6nt9.png)
Where
![[A]_(t)](https://img.qammunity.org/2020/formulas/chemistry/college/3u53pt0ubfgy8uere0zzjh717exzf1y6t6.png) is concentration of A after "t" time and
is concentration of A after "t" time and
![[A]_(0)](https://img.qammunity.org/2020/formulas/chemistry/college/qqxha3gkqyi6nvxca57jmthzzzu8zyjq3q.png) is initial concentration of A
is initial concentration of A
At half-life,
![[A]_(t)=([A]_(0))/(2)](https://img.qammunity.org/2020/formulas/chemistry/college/4jyq4lumzku2hqeegq6kvbela3hlq8j8gb.png)
Here
![[A]_(0)=0.737M](https://img.qammunity.org/2020/formulas/chemistry/college/iw3zwv3nt2xsoywz4s0lxpauqh44zg1rqj.png) and
and
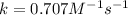
Plug-in all the values in the above equation-
![(1)/(([A]_(0))/(2))=(0.707M^(-1)s^(-1)* t)+(1)/([A]_(0))](https://img.qammunity.org/2020/formulas/chemistry/college/5sfks090bc1xu9a1gch3mkmzd8iagxldq8.png)
or,
![(1)/([A]_(0))=0.707M^(-1)s^(-1)* t](https://img.qammunity.org/2020/formulas/chemistry/college/42tfluibyuinpsi6n4pzlvetu2uns7v48l.png)
or,
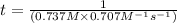
or,
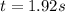
So, half-life for the reaction is 1.92s