Answer:
Wavelength,
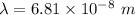
Step-by-step explanation:
It is given that,
The binding energy of an electron is,
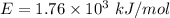
or
The binding energy of an electron is,
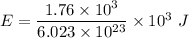
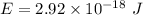
Let
 is the wavelength of light required to remove an electron from the surface of potassium metal by the photoelectric effect. The energy of an electron is given by :
is the wavelength of light required to remove an electron from the surface of potassium metal by the photoelectric effect. The energy of an electron is given by :
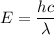
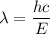
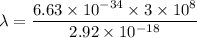
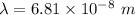
So, the longest wavelength of light required to remove an electron from the surface of potassium metal is
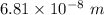 . Hence, this is the required solution.
. Hence, this is the required solution.