Step-by-step explanation:
Since, it is given that both hexane and heptane are miscible with each other. Therefore, a homogeneous mixture will be formed.
As a homogeneous mixture is the mixture formed due to even distribution of solute particles into the solution.
Hence, intermolecular forces present within the molecules will be very small. So, enathlpy of the mixture will be equal to zero. (As for ideal mixture
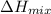 is nearly zero)
is nearly zero)
Due to weak intermolacular forces of attraction the molecules will not mix with each other. Hence, randomness between them increase leading to an increase in entropy.
Thus, we can conclude that for the given solution entropy of the system increased.