Answer:
Rate of reaction will be half of it's initial value
Step-by-step explanation:
For the given
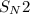 reaction, the rate law is -
reaction, the rate law is -
![Rate=k[1-iodo-2-methylbutane][CN^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/jz5u1zdf7s2wugc8evynu7b3h0uvomssuk.png)
Where k is rate constant, [1-iodo-2-methylbutane] is concentration of 1-iodo-2-methylbutane and
![[CN^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/igpe18vjapuf0wwmd66ksy6yyeliaawqll.png) is concentration of
is concentration of
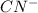
Here nucleophile is the
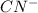 ion
ion
Initiallly,
![(Rate)_(initial)=k* [1-iodo-2-methylbutane]_(initial)* [CN^(-)]_(initial)](https://img.qammunity.org/2020/formulas/chemistry/college/5iwhyy7tyiix1qa0bi5fm3rytaygj71tjz.png)
When concentration of
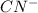 is halved then-
is halved then-
![Rate=k* [1-iodo-2-methylbutane]_(initial)* ([CN^(-)]_(initial))/(2)=((Rate)_(initial))/(2)](https://img.qammunity.org/2020/formulas/chemistry/college/vuf84zy71d5sp0cv770bmvb73urclpv2bn.png)
So rate of reaction will be half of it's initial value