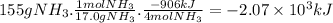Answer:
-2.07 × 10³ kJ
Step-by-step explanation:
Let's consider the following balanced equation.
4 NH₃(g) + 5O₂(g) → 4 NO(g) + 6H₂O(g) ΔHrxn =−906 kJ
The enthalpy of reaction is -906 kJ, that is, 906 kJ are released upon the reaction of 4 moles of NH₃. Taking into account that the molar mass of NH₃ is 17.0 g/mol, the heat associated with the complete reaction of 155 g of NH₃ is: