To solve the problem it is necessary to refer to the definition of entropy.
Entropy is defined aso
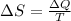
Where,
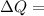 Heat exchange
Heat exchange
T = Temperature
Since the heat exchange is conserved and it is an isothermal process where the temperature remains constant the change in entropy remains the same, ie
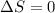 (Reamins same)
(Reamins same)
Therefore the correct answer is C.