Answer:B
Step-by-step explanation:
Option A:
In the nuclear reactions,neutron can be split into proton an electron.
In this case,the number of protons are increasing,increasing the atomic number.
Hence,the atomic number is not conserved.
Option B:
In any reaction the sum of number of neutrons and number of protons remain constant before and after the reaction.
Option C:
The mass number may change for the atoms.For example
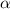 emission reduces the atomic number by
emission reduces the atomic number by
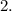
Option D:
In the nuclear reactions,neutron can be split into proton an electron.
So,they number of neutrons may change.