Answer : The correct option is, (E) A pathway by which counterions can flow between the half-cells without the solutions in the half-cell totally mixing.
Explanation :
Salt bridge : It is a type of laboratory device that is used to connect the reduction and oxidation half cell of a electrochemical cell.
The function of salt bridge in the cell is to maintain the electrical neutrality within the internal circuit because the electrons are moving from one half cell to another half cell.
It is made up with salt like
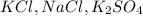
Hence, the correct option is, (E)