Answer:
877
Step-by-step explanation:
P1 = 150 atm
V1 = 0.12 m^3
P2 = 1.2 atm
V2 = ?
radius of each balloon, r = 0.16 m
Volume of each balloon,
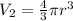
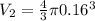
V2 = 0.0171 m^3
Let the number of balloons be N.
So,
P1 x V1 = N x P2 x V2
150 x 0.12 = N x 1.2 x 0.0171
N = 877.19
So, the number of balloons be 877.