To solve the problem it is necessary to consider the concepts and formulas related to the change of ideal gas entropy.
By definition the entropy change would be defined as
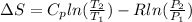
Using the Boyle equation we have
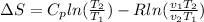
Where,
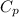 = Specific heat at constant pressure
= Specific heat at constant pressure
 = Initial temperature of gas
= Initial temperature of gas
 = Final temprature of gas
= Final temprature of gas
R = Universal gas constant
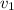 = Initial specific Volume of gas
= Initial specific Volume of gas
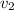 = Final specific volume of gas
= Final specific volume of gas
According to the statement, it is an isothermal process and the tank is therefore rigid
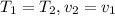
The equation would turn out as
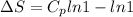
Therefore the entropy change of the ideal gas is 0
Into the surroundings we have that
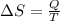
Where,
Q = Heat Exchange
T = Temperature in the surrounding
Replacing with our values we have that
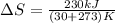
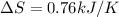
Therefore the increase of entropy into the surroundings is 0.76kJ/K