To develop this problem it is necessary to apply the definitions of entropy change within the bodies
The change of entropy in copper would be defined as
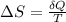
Where,
Q= Heat exchange
T = Temperature
For an incompressible substance, the change in the heat exchange is defined as

Where,
m = Mass
c = Specific heat
Replacing in our equation we have that
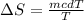
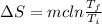
Since
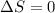 , then
, then
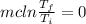
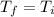
In this way for the change of enthalpy and internal energy you have to
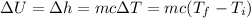
As
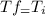 , then
, then

Therefore the correct option is A. No change at All