Answer:C
Step-by-step explanation:
A chemical equation is a equation that describes a corresponding chemical reaction.
A chemical reaction is generally written as
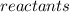 →
→
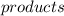
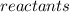 refer to all the reactants involved in the chemical reaction.
refer to all the reactants involved in the chemical reaction.
Reactants are usually written on the left hand side of the chemical equation.
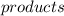 refer to all the products formed in the chemical reaction.
refer to all the products formed in the chemical reaction.
products are usually written on the right hand side of the chemical equation.
In the given reaction,
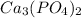 ,
,
 are written on the right side of the equation.
are written on the right side of the equation.
So,
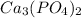 ,
,
 are the products.
are the products.