Answer : The final pressure in the system is 4.22 atm.
Explanation :
First we have to calculate the moles of methane.
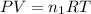
where,
P = pressure of gas = 9.61 atm
V = volume of gas = 3.06 L
T = temperature of gas = T
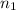 = number of moles of methane gas = ?
= number of moles of methane gas = ?
R = gas constant
Now put all the given values in the ideal gas equation, we get:
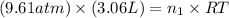
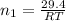
Now we have to calculate the moles of oxygen gas.
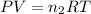
where,
P = pressure of gas = 1.75 atm
V = volume of gas = 6.65 L
T = temperature of gas = T
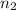 = number of moles of oxygen gas = ?
= number of moles of oxygen gas = ?
R = gas constant
Now put all the given values in the ideal gas equation, we get:
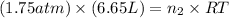
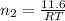
Now we have to determine the final pressure in the system after mixing the gases.
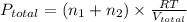
where,
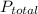 = final pressure of gas = ?
= final pressure of gas = ?
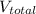 = final volume of gas = (3.06 + 6.65)L = 9.71 L
= final volume of gas = (3.06 + 6.65)L = 9.71 L
T = temperature of gas = T
R = gas constant
Now put all the given values in the ideal gas equation, we get:
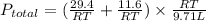
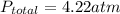
Therefore, the final pressure in the system is 4.22 atm.