Answer:
Q_d=35881 J/mol
So the activation energy is 35881 J/mol.
Step-by-step explanation:
Consider the following equations:
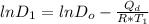
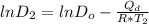
Solving the above two equation to find the Q_d in term of diffusivity and temperature we will get:
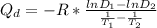
where:
Q_d is the activation energy
D_1 is the diffusivity at T_1
D_2 is the diffusivity at T_2
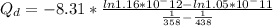
Q_d=35881 J/mol
So the activation energy is 35881 J/mol.