Answer:
the heat absorbed by the block of copper is 74368.476J
Step-by-step explanation:
Hello!
To solve this problem use the first law of thermodynamics that states that the heat applied to a system is the difference between the initial and final energy considering that the mass and the specific heat do not change so we can infer the following equation
Q=mCp(T2-T1)
Where
Q=heat
m=mass=2.3kg
Cp=0.092 kcal/(kg C)=384.93J/kgK
T2=Final temperatura= 90C
T1= initial temperature=6 C
solving
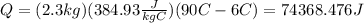
the heat absorbed by the block of copper is 74368.476J