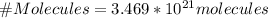To solve this exercise it is necessary to apply the concepts related to Robert Boyle's law where:

Where,
P = Pressure
V = Volume
T = Temperature
n = amount of substance
R = Ideal gas constant
We start by calculating the volume of inhaled O_2 for it:
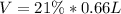
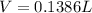
Our values are given as
P = 1atm
T=293K

Using the equation to find n, we have:

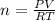
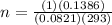
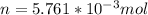
Number of molecules would be found through Avogadro number, then