Answer:
(a) 14 mL
(b) At the equivalence point, the solution will be basic.
Step-by-step explanation:
(a) What volume of 0.15 M KOH would need to be added to 25 mL of 0.086 M lactic acid to reach the equivalence point? (keep 2 significant figures)
Let's consider the neutralization between lactic acid and KOH.
HC₃H₅O₃(aq) + KOH(aq) ⇄ KC₃H₅O₃(aq) + H₂O(l)
+
The molar ratio of HC₃H₅O₃ to KOH is 1:1.
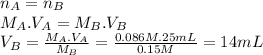
(b) At the equivalence point, would the aqueous solution be acidic, basic, or neutral? Explain why.
At the equivalence point, the solution will be basic. At this stage, all of the lactic acid in the solution will have reacted with the KOH added, producing lactate ions (C₃H₅O₃⁻) and potassium ions (K⁺) in the solution. The potassium ions will not affect the pH (it comes from KOH, which is a strong base), but the lactate ions will make the solution basic due to the basic hydrolysis, as follows:
C₃H₅O₃⁻(aq) + H₂O(l) ⇄ HC₃H₅O₃(aq) + OH⁻(aq)