Answer: The true statements are following:
- Energy of the universe is constant; the entropy of the universe increases.
- If a process increases the randomness of the particles of a system, the entropy of the system increases.
- All spontaneous processes release heat.
- Both
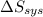 and
and
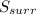 must equal zero at equilibrium.
must equal zero at equilibrium.
Step-by-step explanation:
Spontaneous reactions are defined as the reaction which occur on its own and they do not require any external force to start.
- All spontaneous reactions show a positive value for change in entropy. Each spontaneous reaction requires different amount of time.
Hence, the statement all spontaneous reactions occur quickly, is false.
- Entropy of the Universe always increases and energy always changes from one form to another. Hence, the statement energy of the universe is constant; the entropy of the universe increases, is true.
- As entropy is the degree of randomness. So, with increases the randomness of the particles of a system there will also be increase in randomness.
Therefore, the statement if a process increases the randomness of the particles of a system, the entropy of the system increases, is true.
- Some processes are non-spontaneous in nature. Hence, the statement all systems become more disordered spontaneously, is false.
- Since, randomness between the molecules increases so, heat is eventually released in all spontaneous processes. Therefore, the statement all spontaneous processes release heat, is true.
- For a reversible process, entropy change of the Universe is equal to 0. Therefore,
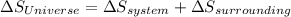
Hence, the statement both
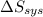 and
and
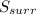 must equal zero at equilibrium, is true.
must equal zero at equilibrium, is true.