Answer : The heat of combustion of n-propanol is 0.554 kJ/mol
Explanation :
First we have to calculate the moles of n-propanol.
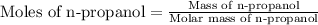
Molar mass of n-propanol = 60.09 g/mole
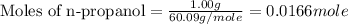
Now we have to calculate the heat of combustion of n-propanol.
As, 0.0166 mole of n-propanol liberated heat of combustion = -33.4 kJ
So, 1 mole of n-propanol liberated heat of combustion = 0.0166 × (-33.4 kJ)
= 0.554 kJ/mol
Therefore, the heat of combustion of n-propanol is 0.554 kJ/mol