Answer:
pH is closest to 4.0
Step-by-step explanation:
According to Henderson-Hasselbalch equation for a buffer consist of an weak acid (HA) and it's conjugate base (
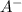 )-
)-
![pH=pK_(a)(HA)+log([A^(-)])/([HA])](https://img.qammunity.org/2020/formulas/chemistry/high-school/inrc8h1fm7zh0kaybrghz2apy10klxjdrp.png)
where
![[A^(-)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/96pqc72ce8imq1w12ml3gyiqb09mdzzviw.png) and [HA] represents concentration (in molarity) of
and [HA] represents concentration (in molarity) of
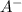 and HA respectively.
and HA respectively.
We know,
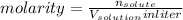
Number of moles of HA and
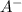 remains same even after diluting the solution to 10 L.
remains same even after diluting the solution to 10 L.
So,
![([A^(-)])/([HA])=((n_(A^(-)))/(V_(solution)in liter))/((n_(HA))/(V_(solution)in liter))=((1 mol)/(10 L))/((1mol)/(10L))=1](https://img.qammunity.org/2020/formulas/chemistry/high-school/jmqev0nod2vwuf461zot4cgi6d757l63oz.png)
So,
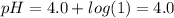
Hence pH is closest to 4.0