Answer:
A) 2.27 grams of tungsten trioxide must be needed to prepare 1.80 g of tungsten.
B)To prepare 1.80 g of tungsten we will need 0.0587 grams of hydrogen gas.
Step-by-step explanation:
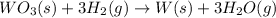
A) Mass of tungsten prepared = 1.80 g
Moles of tungsten =
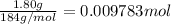
According to reaction, 1 mol of tungsten is obtained from 1 mole of tungsten trioxide.
Then 0.009783 moles of tungsten will be obtained from:
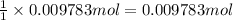 of tungsten trioxide
of tungsten trioxide
Mass of 0.009783 moles of tungsten trioxide :
0.009783 mol × 231.8 g/mol = 2.27 g
2.27 grams of tungsten trioxide must be needed to prepare 1.80 g of tungsten.
B) According to reaction,1 mol of tungsten is produced by 3 moles of hydrogen gas
Then 0.009783 moles of tungsten are produced by :
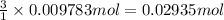
Mass of 0.02935 moles of hydrogen gas:
0.02935 mol × 2 g/mol =0.0587 g
To prepare 1.80 g of tungsten we will need 0.0587 grams of hydrogen gas.