Step-by-step explanation:
The given data is as follows.
Concentration = 3.3 mol
Rate constant =
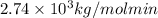
Molecular weight = 55000 g/mol
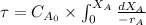
Initial concentration (
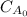 ) of aminoheptanoic is 3.3 kg/mol.
) of aminoheptanoic is 3.3 kg/mol.
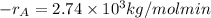
Hence, putting the given values into the above formula as follows.
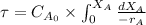
=
![(3.3)/(2.74 * 10^(3) kg/mol min) [X_(A)]^(0.1)_(0)](https://img.qammunity.org/2020/formulas/chemistry/college/c83wqg5n1qzew4imbnlqdwonurhf02c74a.png)
= 120.43 min
Thus, we can conclude that total time required to to form polyamide is 120.43 min.