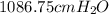Answer:
Barometric pressure will be
Step-by-step explanation:
We know,
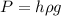
where P is barometric pressure, h is height of liquid inside barometer,
 is density of liquid and g is gravitational constant
is density of liquid and g is gravitational constant
If barometric pressure is x cm
 for 805 mm Hg then-
for 805 mm Hg then-
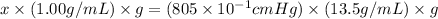
So,
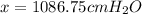
Hence barometric pressure will be